Labeling & Testing for Medical Cannabis
People often ask me why I expanded into medical cannabis from pharmaceuticals and medical devices. Well, it comes back to one word…opportunity. Cannabis is a product that helps people and is used all over the world to treat diseases and illnesses. The difference between this and a pharmaceutical product is that one is natural and one is synthetic. This is why you are starting to see similarities often seen in the pharmaceutical industry: proper packaging and labeling, ISO certified labs, and Good Manufacturing Practices are only a few of the commonalities across both.

Colorado went even further than most other states when it comes to mimicking expectations of pharmaceutical products. They have introduced “validation” as a way to reduce the testing burden for cannabis processors. Validation is used in manufacturing for pharmaceuticals, medical devices, and now cannabis in lieu of verification – the need to sample every batch of product every time.
Here is a breakdown of the differences between validation and verification:
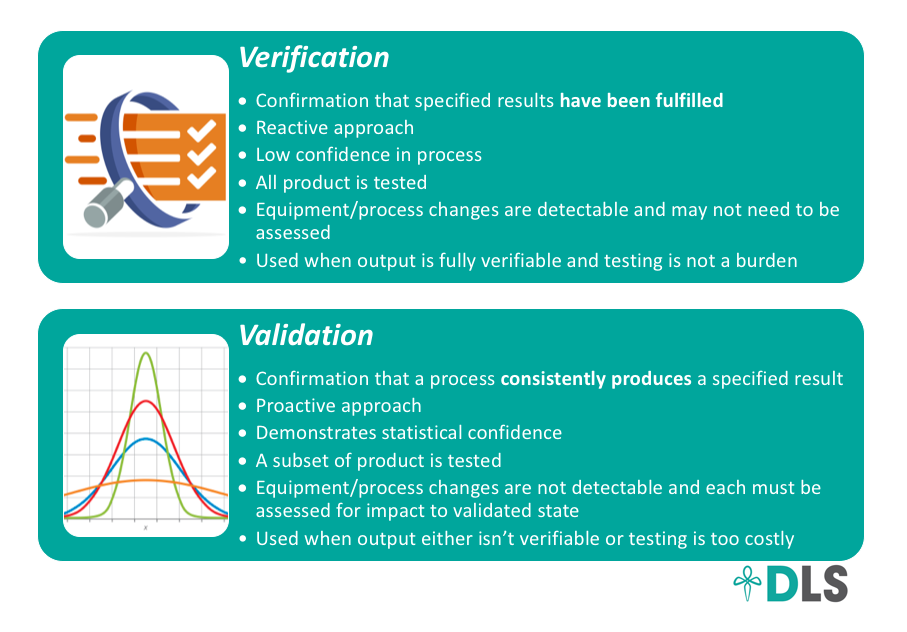 Validation can result in a huge cost savings but you have to ensure that a robust quality system is in place to detect changes to your validated state. For example, how are you going to determine if your new extraction equipment results in the same product quality? How you document, track, and assess changes to your processes will help you determine that. Colorado has done a great job requiring that quality controls such as this are in place for processors who seek to use validation to reduce the amount of testing they have to perform.
Validation can result in a huge cost savings but you have to ensure that a robust quality system is in place to detect changes to your validated state. For example, how are you going to determine if your new extraction equipment results in the same product quality? How you document, track, and assess changes to your processes will help you determine that. Colorado has done a great job requiring that quality controls such as this are in place for processors who seek to use validation to reduce the amount of testing they have to perform.
What is on the label?
- License #
- Batch #
- Net weight or volume
- % total CBD and THC
- Date of sale
- Solvent list
- Ingredients list
- Expiration or use by date (edibles and skin/body products only)
- Production date (skin/body products only)
- Warning statements
How is product quality ensured?
- Every batch is tested for cannabinoid profile (THC, THCA, CBD, CBDA, CBN), heavy metals, solvents, microbials, pesticides, mycotoxins if not validated
- Batches are destroyed if testing fails (retesting is allowed)
- State performs periodic planned and unplanned inspections
- State published list of pesticides/chemicals that are banned from use
- Proficiency testing required for labs
Strengths
- Changes to materials, equipment, and processes must be re-validated
- Retesting is allowed and must be verified by two different test labs prior to sale – both samples must pass
- State requires firms evaluate and destroy or re-process full batches upon validation failure
Flaws
- Moisture content or water activity not required to be tested
- Solvents are required on the label even though they should have been removed from the product
- No mention of recalling batches already in retail if a validation batch fails
Source: https://www.sos.state.co.us/CCR/GenerateRulePdf.do?ruleVersionId=7808&fileName=1%20CCR%20212-2
